About cookies on this site Our websites require some cookies to function properly (required). In addition, other cookies may be used with your consent to analyze site usage, improve the user experience and for advertising. For more information, please review your options. By visiting our website, you agree to our processing of information as described in IBM’sprivacy statement. To provide a smooth navigation, your cookie preferences will be shared across the IBM web domains listed here.
Healthcare
Streamlining Life Sciences Research: Simplifying Data Access with Design Principles
26 March, 2024 | Written by: Dr. Nicole Mather and Seema Raman
Categorized: Healthcare
Share this post:
Life Sciences research and development is complicated: complex tasks are carried out in an environment of big data, specialised technology and regulated processes. The challenges of this complexity risks making processes and interactions iterative, slow and expensive. To accelerate innovation in the development of safe and effective treatments, Life Sciences organisations need to cut through these challenges and move faster. The discipline of Design Thinking can provide a step change in the approach to help us accelerate processes behind the innovation of new treatments and significantly reduce the time to market.
When access to complex analytics tools is provided in a user-friendly way, improving the experience of accessing data – see ChatGPT – the speed of uptake can be exponential. It took ChatGPT just two months to reach 100 million users, redefining the benchmark for digital adoption. Delivering analytical services that are this easy to use is challenging but we believe that a focus on accessibility is critical to unlocking the value from all kinds of data and processes.
Design Thinking is a problem-solving method that puts human needs and experiences at the centre of the design process. It is an approach used to tackle complex problems and generate faster solutions that are user-centric and empathetic. We are seeing increasing numbers of life sciences organisations turning to Design Thinking as a way to create end-to-end digital experiences that better meet the specialist needs of scientists and researchers interacting with data. Designing in collaboration with users, and giving them the last word, is a powerful tactic to ensure solutions fit into users’ business processes and exceed expectations.
Design Thinking enables digital transformation and elevates the experience of a user’s day-to-day activities. This enables co-creation to unlock creativity, bridging the gaps between the world of art and science. Taking a user-led approach can uncover the goals of scientists and understand current processes and challenges – this can help to identify the data needed to support goals and design solutions and intelligent workflows that harmoniously augment scientists’ analytics-based activities and decision-making.
Design Thinking Approach
We work at the intersection of strategy, creativity, and technology to help our clients to digitally reinvent their businesses. Through delivery of innovative, research-driven solutions, we have a track record of successfully addressing complex challenges by rethinking how design is done in most industries, including Life Sciences.
In order to bring together user-centred design and technical development we have created an approach called IBM Garage. Our teams have been at the forefront of bringing IBM Design Thinking together with Science and Research. This has allowed us to deliver user-centred data and technology transformation that puts scientists at the heart of any solution.
Design Thinking can be a novel experience for some of our clients, but our expertise in the Life Sciences industry has enabled us to apply this approach with scientific stakeholders in a way that delivers value faster than many of our clients expect. Our deep industry experience allows us to effectively translate and prioritise clinical requirements and workflows to reimagine experiences and improve scientific outcomes. It is an empowering and energising experience to design and deploy new approaches in such a fast-paced way.
A few stories of Design Thinking in Life Sciences:
Genomics Organisation
IBM was engaged as a delivery partner to support the design and build of an innovative new portal to support the diagnosis of cancers.
We mobilised a joint team with experience in science and genomic, agile delivery methods including design thinking, user-centered design, software development and AWS cloud. The client teams brought deep expertise and insights into genomic data analysis and interpretation, bioinformatics and day to day processes plus complex programme delivery.
Over 16 months, the team co-designed and built an innovative system capable of whole genome sequence analysis that delivers an exceptional clinical user experience, expedites cancer diagnosis and is based on a modern, secure, flexible model for the future.
Our overall approach and architecture aligns with three guiding principles:
- Value-based: all recommended solutions are associated with user benefits
- User-experience: technologies to support clinical scientists in rapid diagnosis
- Secure: open cloud platform prioritising data security, governance as code, and resilience
This delivered a solution with user-friendly front-end design, combining five clinical screens into one, reducing cognitive load and allowing users to focus on clinical decisions. Along with APIs to support data filtering and aggregation this enabled a highly-performant approach to surfacing data based on an AWS cloud platform.
‘This is an excellent example of how blended teams can collaborate to solve user needs elegantly and responsively for our clinical colleagues and will have an ongoing positive impact on patient care and lab efficiency’. Deputy Chief Medical Officer
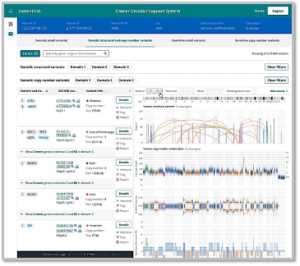

Above are examples of work on the Clinical Variant interpretation for cancer diagnosis
Leading Foundation trust
This major teaching hospital is at the forefront of the UK’s delivery of genomic medicine. Following a successful PoC developed via user-centred design methodologies and working closely with clinicians and researchers, the MVP release of this new digital genomics management service will accelerate treatment for over 5 million people.
This platform allows clinicians to manage genomic patient records and builds the foundation for data science and machine learning to accelerate multi-disciplinary review of patients so that they can be diagnosed faster. The teams made innovative developments to reduce clinical risk by allowing clinicians to learn from patients with a similar history, as finding similar records was accelerated from weeks to seconds. A clear roadmap was also developed for moving from a paper-based system to a fully digitised platform, enabled with machine learning, delivering actionable clinical insights and outcomes.
Clinical teams
We have been engaged in two projects with a leading Clinical Unit both of which have embedded design thinking practices to enhance business and user outcomes. We employed service design to radically rethink a future-state employee and clinical trial volunteer experience that better enables recruitment to efficiently attract, recruit and retain the right volunteers for Phase 1 clinical trials. As a result, we delivered a future-state blueprint, a capability model, underpinned by a value-driven roadmap, for the unit’s digital transformation, to realise the newly designed end-to-end clinical trial volunteer and employee experience.
We then worked to bring the first milestone to life: a modernised clinical trial volunteer website. To achieve a compelling initial volunteer touchpoint, IBM delivered:
- Designed directed-volunteer journeys built on design patterns, with complementary business capabilities
- Trust-building and engaging content through a new website
- Enhanced website functionality to allow volunteers to be more proactive in their applications to clinical trials
Through effective user research and design, the team has been able to make a daunting clinical trial experience feel more familiar to prospective volunteers and accelerate recruitment activities.

Examples of User personas for clinical trial recruitment
Sample management
We supported the delivery of an Enterprise Sample Management solution for a leading biopharma. We deployed user-centred design to understand our clients’ pain points and challenges, and were then able to design and support implementation of a holistic and end-to-end biological sample lifecycle tracking system, which allows the seamless movement of both clinical and non-clinical samples between research units and logistic hubs. Navigating complications in sample and data consent, we worked with researchers to map the current processes of sample transfer today, to create a vision for the future.
We are a key partner to implement a new sample biobank facility, requiring a fully operational sample repository for storage, management and histological processing of all samples in 2024. By leveraging tools from IBM Garage, we have identified and transformed their scientific business requirements into technical capabilities, supporting their innovation and growth towards a technology and data driven organisation.

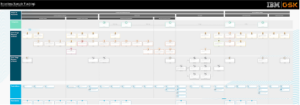
Example to-be service blueprints and end-to-end journey maps for sample transfer and tracking
Summary
A user-centred approach is critical for Life Sciences organisations to become more agile and globally competitive. By designing and creating solutions that meet specific high-value, prioritised needs of scientists and researchers – such as visualisation of complex data, automating and implementing intelligent workflows and applying a strategic approach to data insights – organisations can realise significant benefits. The application of this approach can accelerate research and trials, optimising and accelerating pharmaceutical processes, personalising patient engagement, and providing radically improved data portals for clinicians. There is a significant opportunity for Life Sciences organisations to unlock creativity with their scientists, and accelerate their operations by combining design with digital transformation.
Acknowledgements
The authors would like to thank the team for their significant contributions to this paper: Karishma Govind, Service Design Director; Ravi Benitez, Senior Service Designer; Catherine Heylings, Life Sciences Experience Consultant; Emily Ginn, Agile Delivery Business Analyst, Life Sciences; Chhaya Shah, HLS Intern.

Dr. Nicole Mather
Life Sciences Lead, HLS Data & AI Lead. IBM Consulting UK & Ireland.

Seema Raman
Associate Partner - Healthcare and LifeSciences
More Healthcare stories
By Col Chambers and Ed Gillett on 5 February, 2025
Preparing for the defence of the Realm
In light of current conflicts, the UK is now faced with real-world military decisions that will affect our immediate future. Ed Gillett and Col Chambers assert that industry and government must switch to a readiness mindset before the European post-war peace shatters. “My vision for the British Army is to field fifth-generation land […]
By Juan Bernabe Moreno and others on 12 December, 2024
Frontier Fusion: Accelerating the Path to Net Zero with Next Generation Innovation
Delivering the world’s first fusion powerplants has long been referred to as a grand challenge – requiring international collaboration across a broad range of technical disciplines at the forefront of science and engineering. To recreate a star here on Earth requires a complex piece of engineering called a “tokamak” essentially, a “magnetic bottle”. Our […]
By Nick Levy on 9 December, 2024
Safer Technology Change in the Financial Services Industry
Many thanks to Benita Kailey for their review feedback and contributions to this blog. Safe change is critical in keeping the trust of customers, protecting a bank’s brand, and maintaining compliance with regulatory requirements. The pace of change is never going to be this slow again. The pace of technology innovation, business […]





























